2020 is a year that is hard to be forgotten. The challenges of COVID-19 have had a major impact on everyones life.
Many holidays were cancelled, many people lost their jobs due to the pandemic and the mental health of many others has been affected like never before.
This month the vaccines that have promised more than 90 % effectiveness were Pfizer and Moderna. The groundbreaking news came as a surprise for many people who hope the path to “normality” is near.
This also makes many people more optimistic about how they will celebrate Christmas with their loved ones.
Surprisingly, this week two more vaccines announced their success in trials- the Oxford vaccine AstraZeneca and the Russian vaccine Sputnik V.
On November, 23 it was reported that the Oxford vaccine AstraZeneca revealed 62% or 90 % effectiveness depending on the doses. A few days later the Russian vaccine Sputnik V also notified 90 % vaccine efficacy.
Their efficiency results increased in just a couple of days. Please see more information on the infographics below.
What are the similarities of Covid 19 vaccines?
1. All vaccines need to be kept in a cold environment.
2. All vaccines require two shots a few weeks apart.
3. Moderna and Pfizer vaccines are based on mRNA formula.
RNA vaccines are very easy to be produced only by using a pathogen genetic code.
These vaccines work by inserting into the body a messenger RNA sequence which consists of genetic instructions for the vaccinated individuals’ cells to construct antigens and to achieve an immune response.
4. AstraZeneca and Sputnik V vaccines are affordable. They will cost no more than a few pounds.
5. Both AstraZeneca and Sputnik V vaccines use a type of adenovirus as a base.
The Oxford vaccine, for example, uses a weakened type of common flu.
When the vaccine invades the body’s cells, it will produce “spike protein“ of coronavirus.
Additionally, the vaccine uses chimpanzee adenovirus.
The Sputnik V, on the other side, uses the human adenoviral vector-based platform. This vaccine uses “vectors“-vehicles which can bring a genetic material from another virus to the necessary cell.
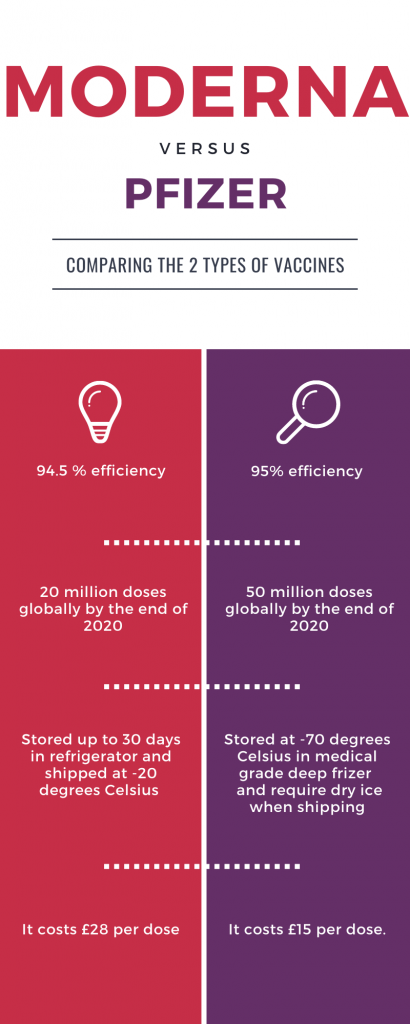
What are the differences between the vaccines?
On the infographics below VoL have described a few differences between the vaccines:
Challenges of the vaccines
Although more of the trials look promising there are many challenges that the vaccines have to overcome.
Vaccines are one of the most significant public health measures which are a central part of the health policy of every country.
However, they take years to be produced with an average expectancy of 15 years. We have already four vaccines but its vital to work around them and prove their safety.
The author Tri Wibawa highlights in the medical review “Covid-19 vaccine research and development: ethical issues” the following concerns:
1. It is important that people don’t get infected with pathogens also known as viruses.
There is still a lack of information in regards to pathogenesis and the predictive place of vaccines while examining people who got infected with coronavirus.
Adverse effects shouldn’t be tolerated because this could risk the health of many people.
The most important conditions for a vaccine to be effective are the following: safety, efficacy and quality.
2. mRNA technology might not be harmless
Most of the governments will like to push the mass production of the vaccines and rely on limited data of promising vaccine candidates.
For example, some of the vaccines use mRNA technology which can lead to several risks such as inflammation and autoimmune conditions.
There are no mRNA-based vaccines that are authorized and used in the market.
3. Lack of animal model and the need for controlled challenge test on people
A significant step of every vaccine development is the need of a challenge test.
The test is a part of the preclinical research in an animal model.
Since the animal model is not available in Covid-19 testing, there is a suggestion such as human challenge testing to be implemented instead.
The testing will include controlled human infection used on volunteers. Although it is possible , it is highly unsafe for the participants.
In the past the controlled human infection was used in vaccine development of malaria, cholera and typhoid. These diseases had established treatment and in the case of coronavirus there is no treatment procedure whatsoever and if something goes wrong the healthy results for the volunteers might be dangerous.
Although most of the vaccines data promises positive outcomes, none of their trials have been published in peer reviewed journals and been assessed by age group, gender or duration of protection.
While examining the common feature of the vaccines it is interesting to emphasize that the way every body will respond to the vaccine is different and the possible side-effects of the vaccines should be carefully assessed and published.
A recent example is the abnormal reaction of a volunteer in India after he received the AstraZeneca vaccine.
A senior official at AstraZeneca said on Sunday that even after the adverse effects, there is no reason to halt the trials.#AstraZeneca #Oxford https://t.co/Ds8endzitg
— WION (@WIONews) November 29, 2020
Finally, many scientists warn that zoonotic coronaviruses will continue to be a permanent threat to society due to its constant changing of nature.
The new strains of the virus are changing and even the new vaccines might not be efficient in a couple of months.
Words by: Vanya Petyova | Subbing: Grace Staley